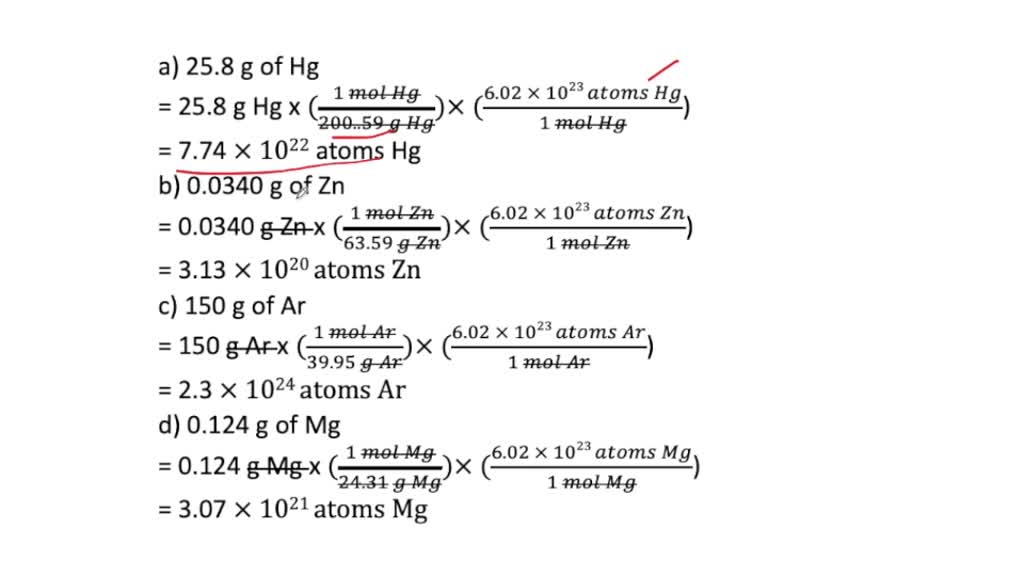
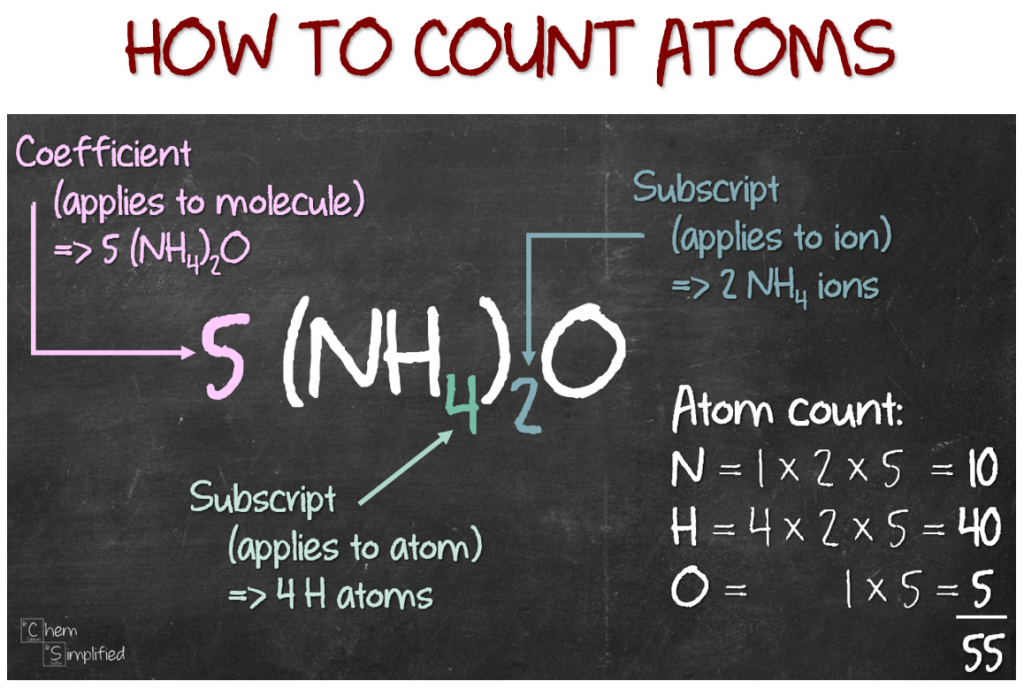
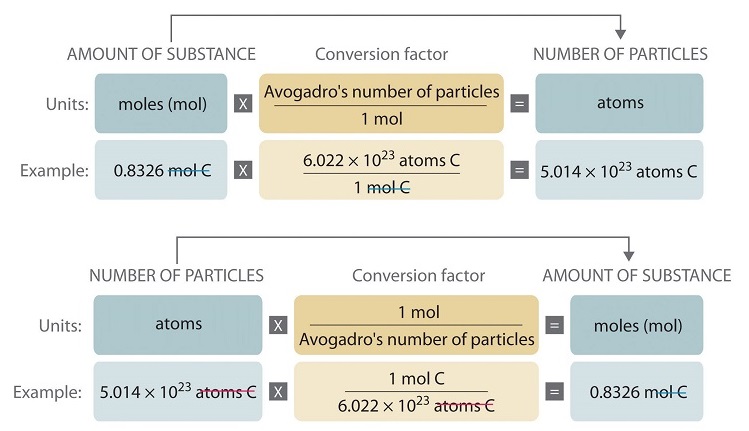
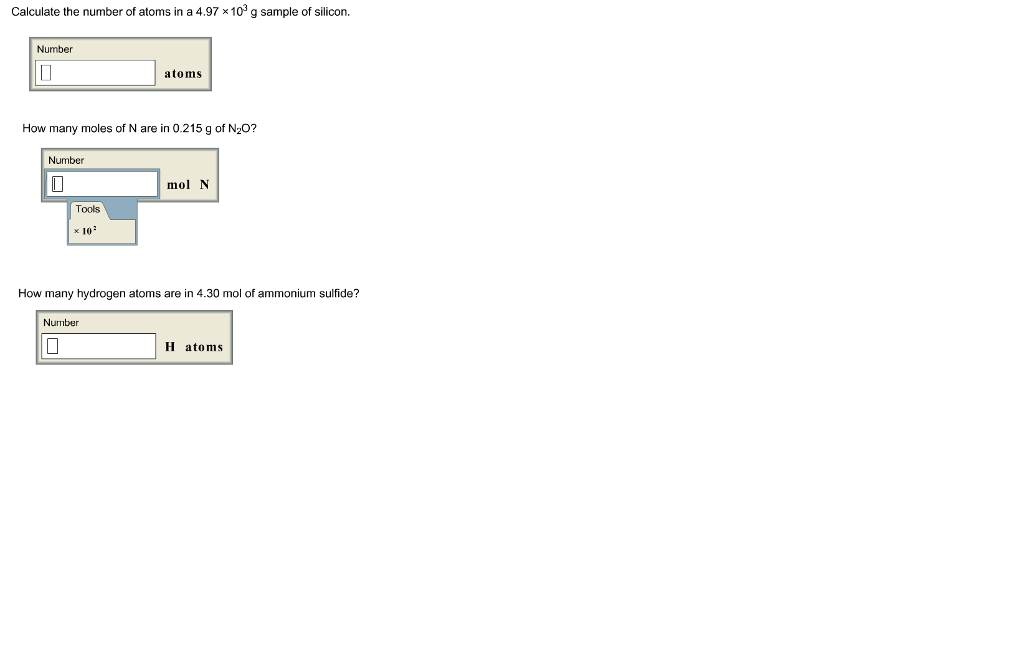
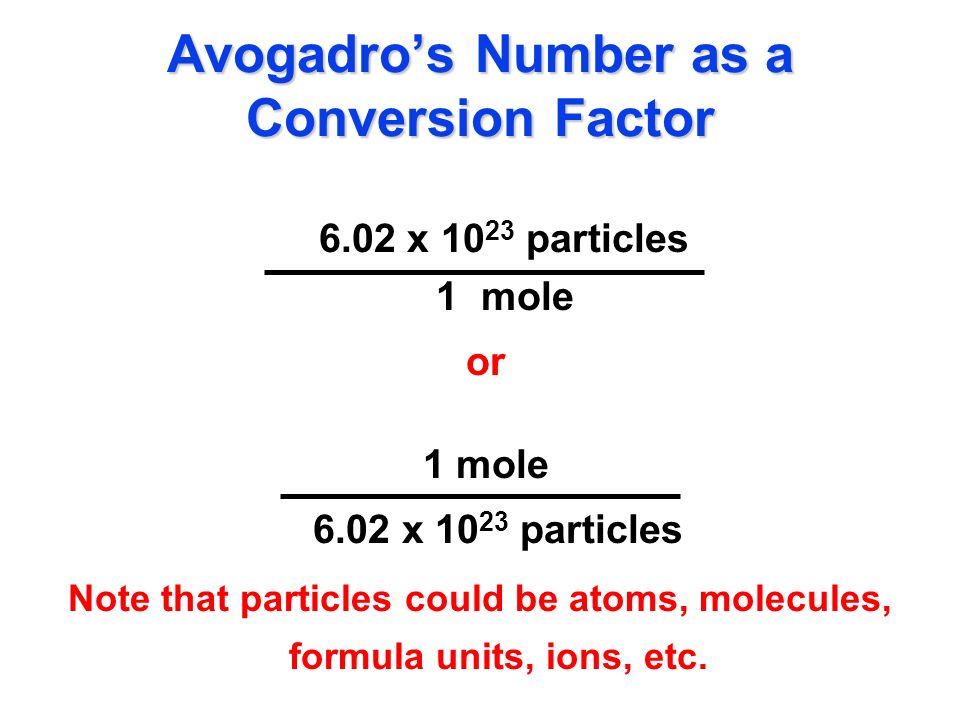
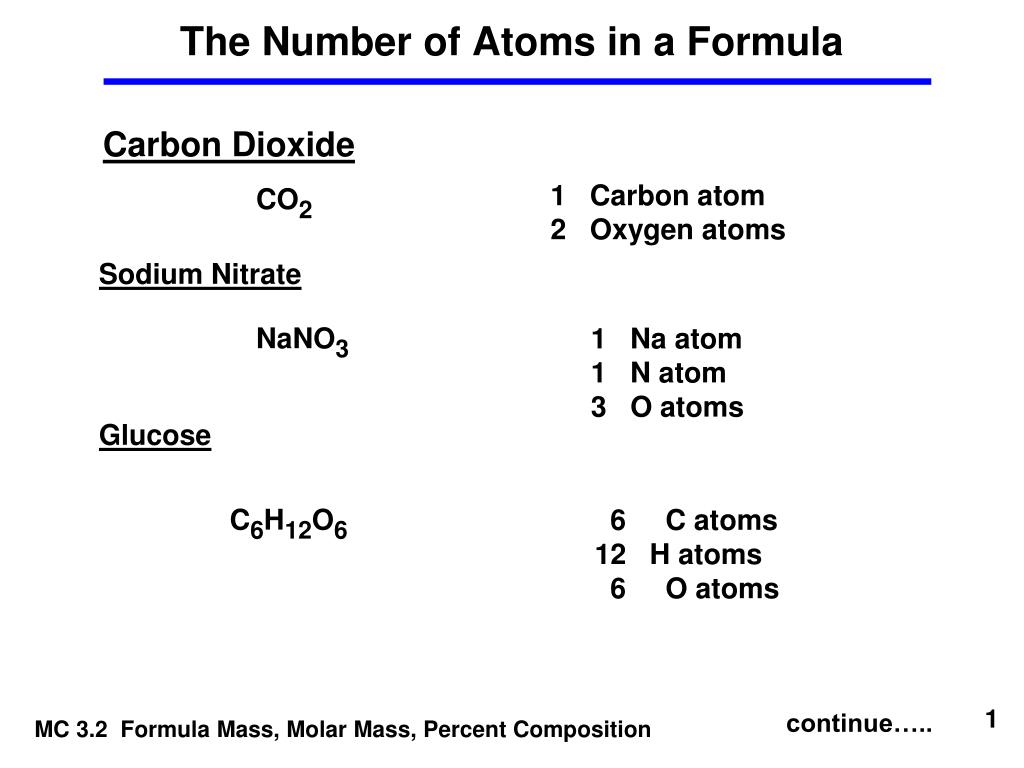
Calculate the number of particles in each of the following: (a) 48 g of Mg (b) 8 g of O2 (c) 0.1 mole of carbon (Atomic mass Mg = 24 u, O =

Calculate the number of atoms present in 2 gram of crystal which has face centred cubic (FCC) crystal lattice having edge length of 100 pm and density 10gcm^-3

Determining the Relative Number of Atoms in a Substance using Elemental Analysis | Chemistry | Study.com
Calculate the number of atoms of each element in 3.42 g of sucrose (C(12)H(22)O(11)) and the total number of atoms.
calculate number of atoms present in 11.2 L ammonia at 273 degrees celsius and 2 atm. a:6.023*10^(23) b:2*6.023*10^(23) c:0.5*6.023*10^(23)