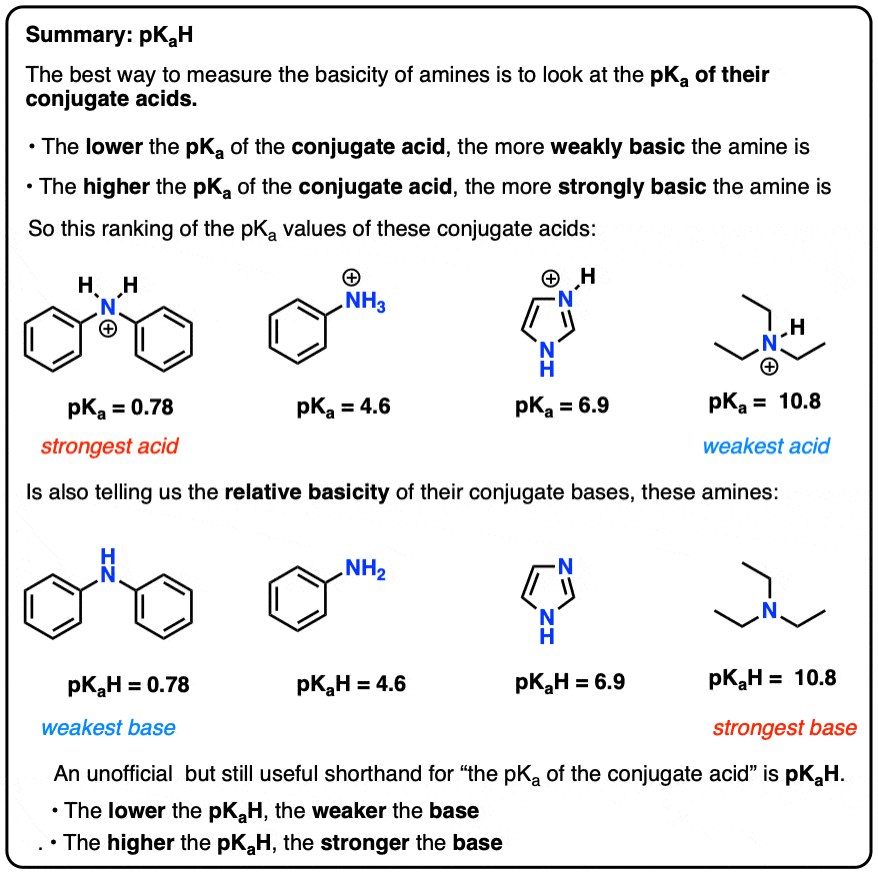
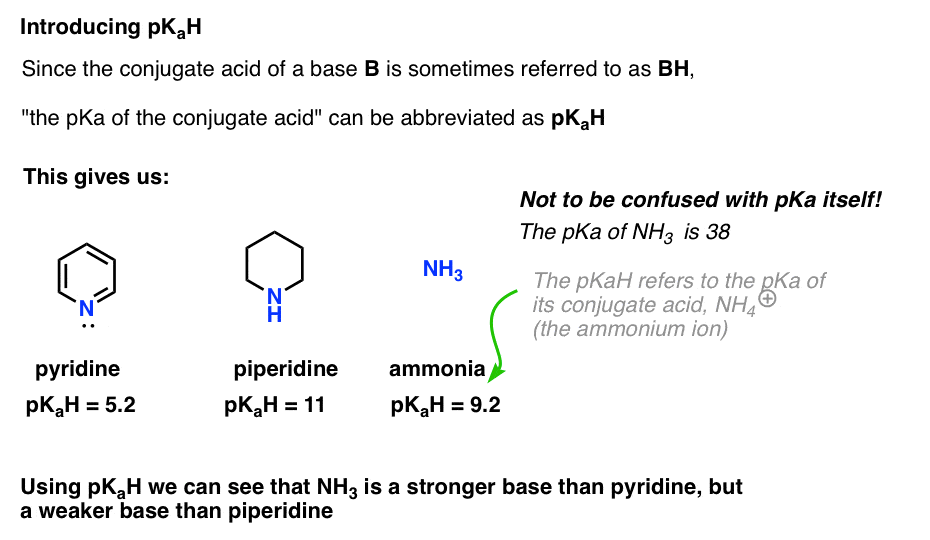
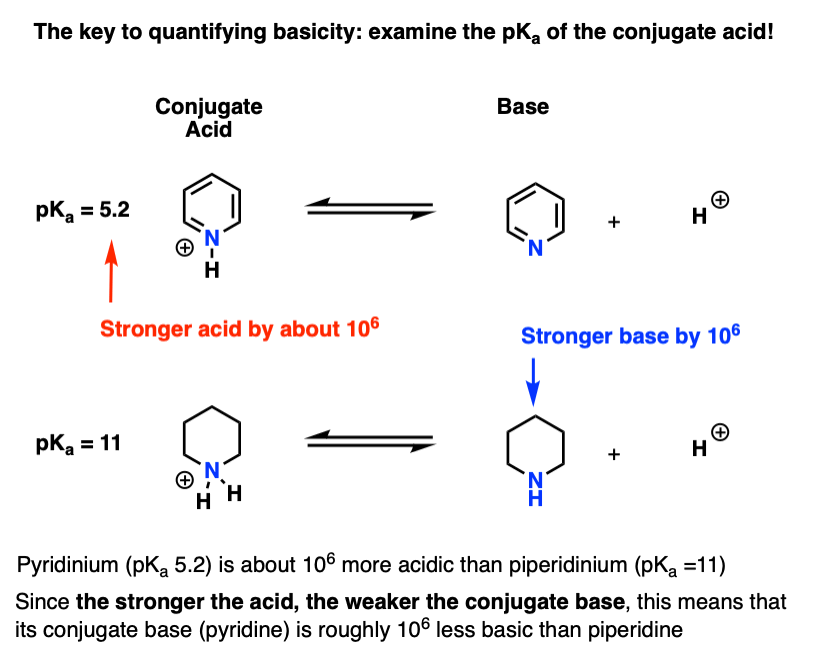
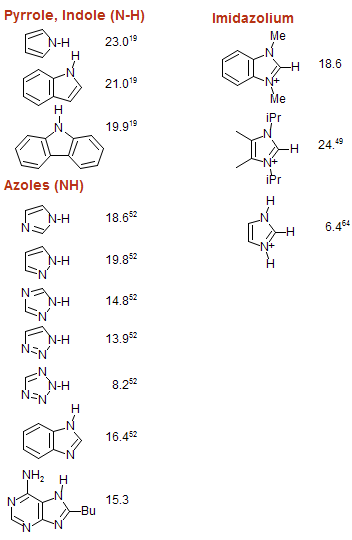
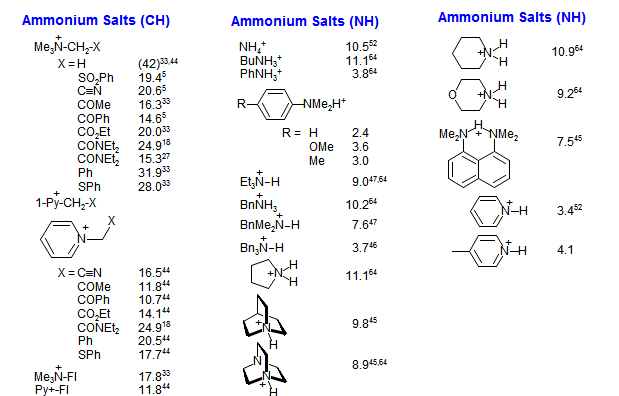
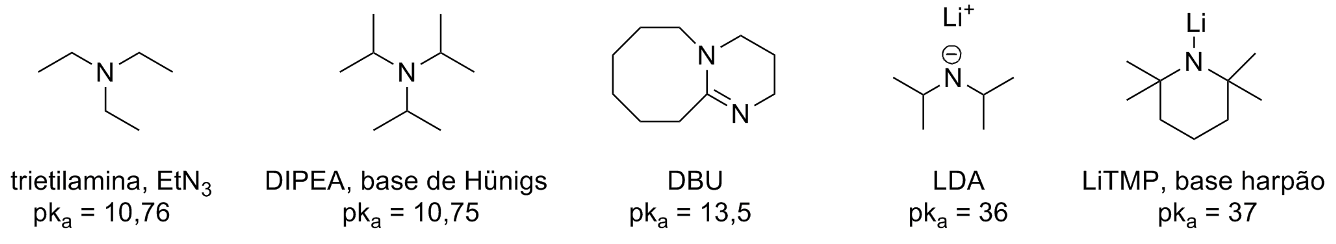
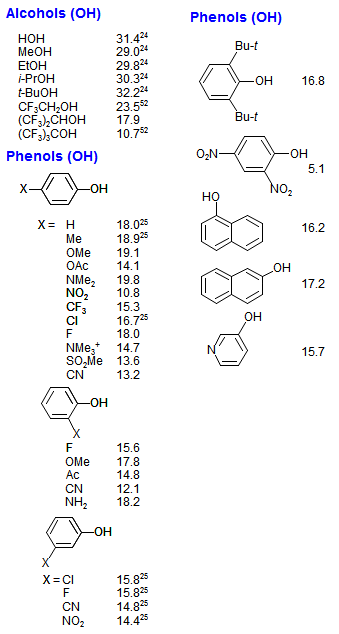
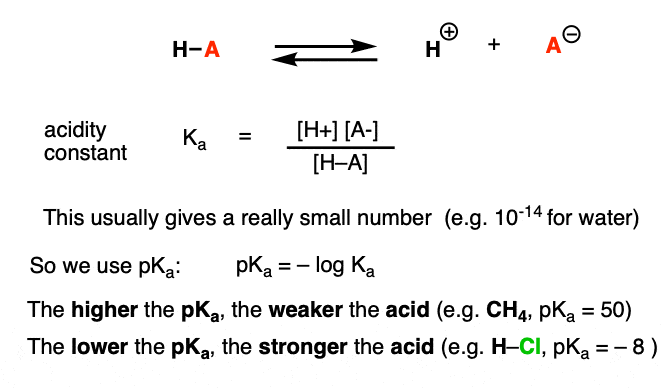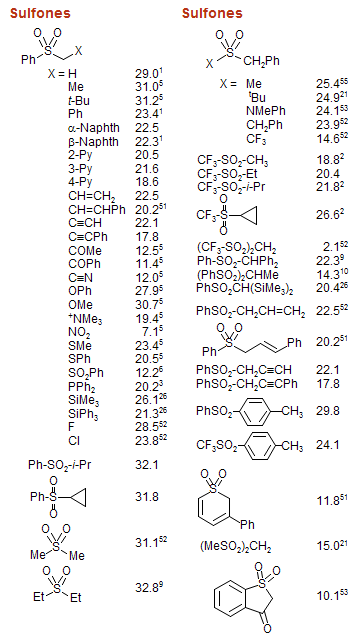Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis

Large-Scale Amidations in Process Chemistry: Practical Considerations for Reagent Selection and Reaction Execution | Organic Process Research & Development

Novel N-Linked Aminopiperidine Inhibitors of Bacterial Topoisomerase Type II with Reduced pKa: Antibacterial Agents with an Improved Safety Profile | Journal of Medicinal Chemistry
Dissociations of free radicals to generate protons, electrophiles or nucleophiles: role in DNA strand breaks