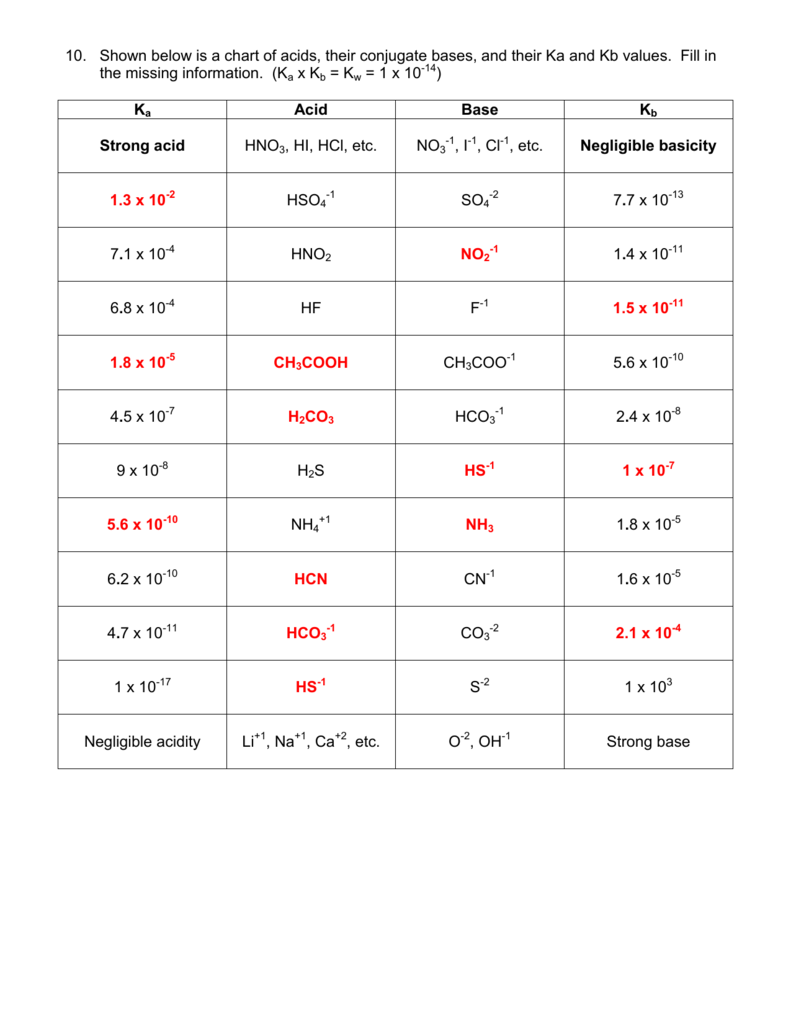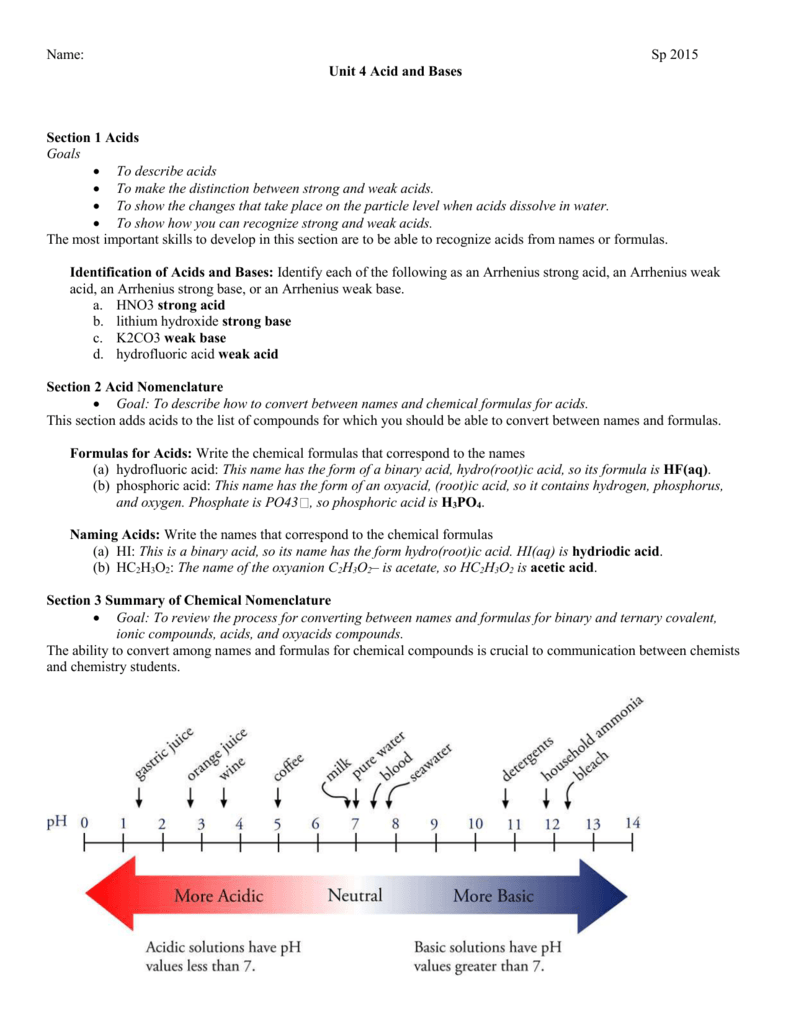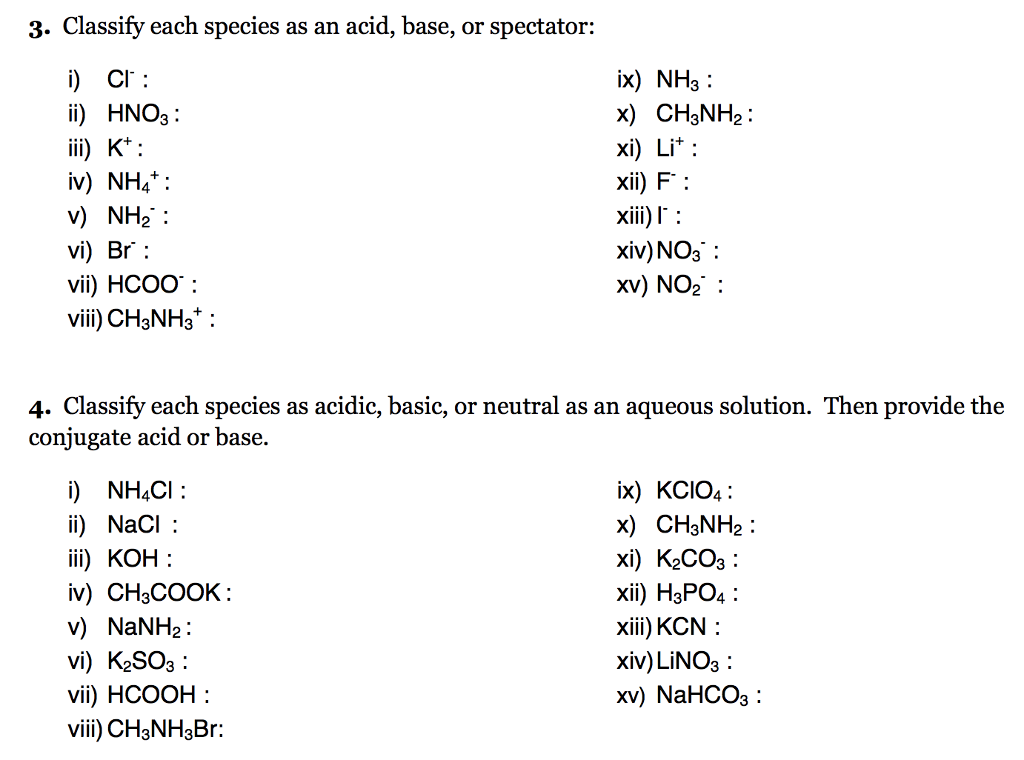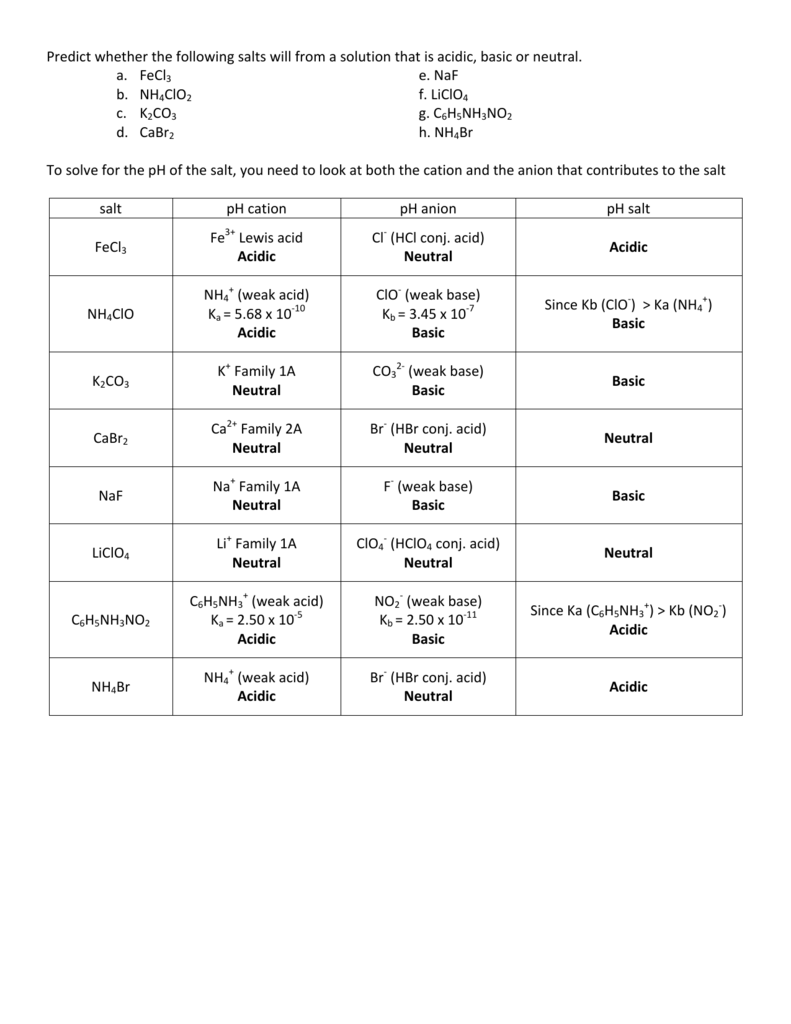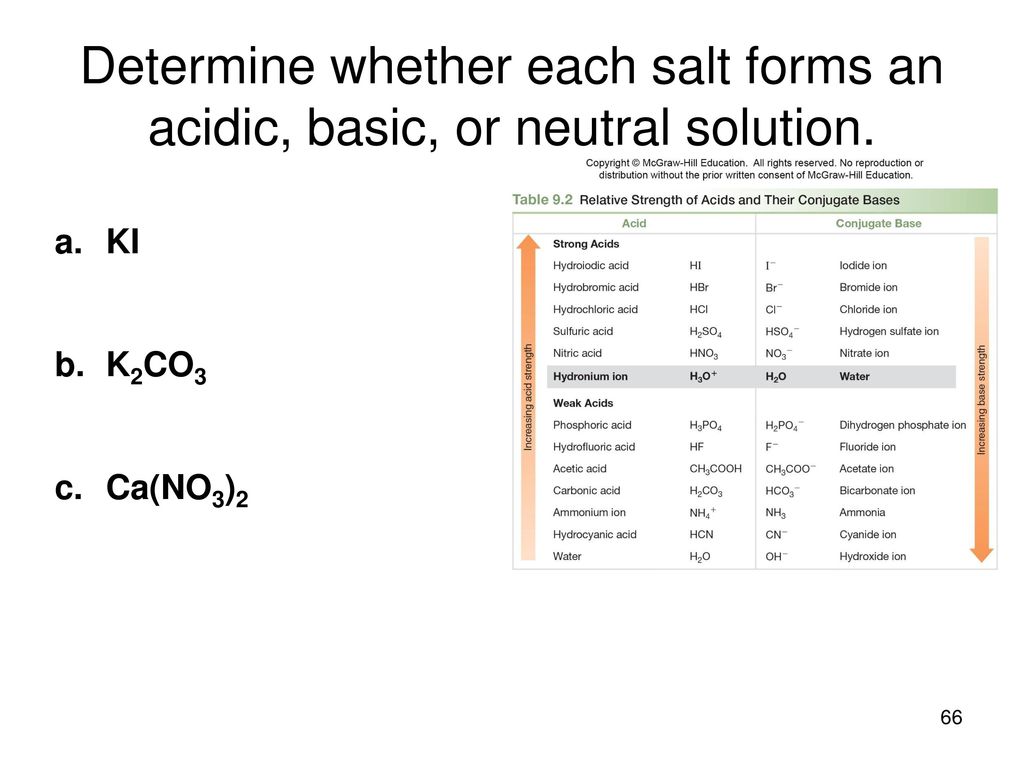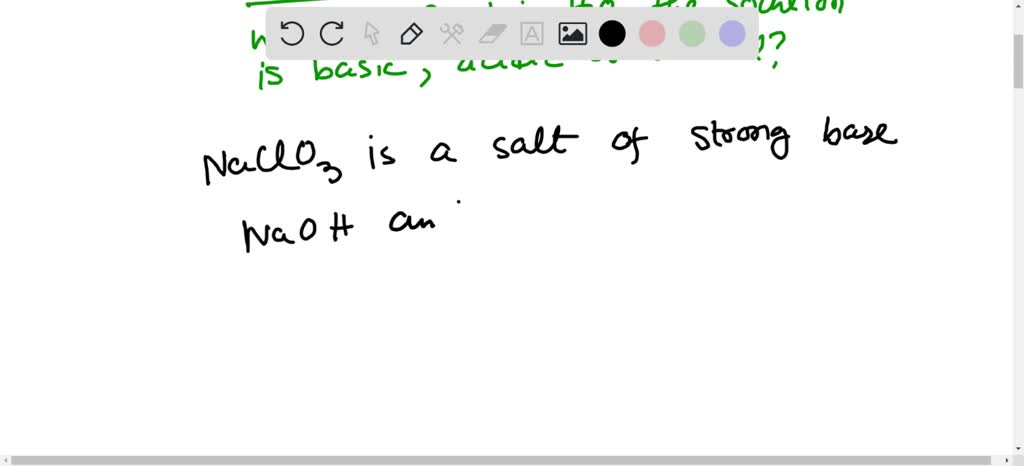
SOLVED: When K2CO3 is dissolved in water, will the solution be acidic, basic, neutral, or will more information be needed to decide?

Classify these salts as acidic, basic, or neutral. And Why? NH4ClO4 , KCl , LiNO3 , NaCN , K2CO3 - YouTube
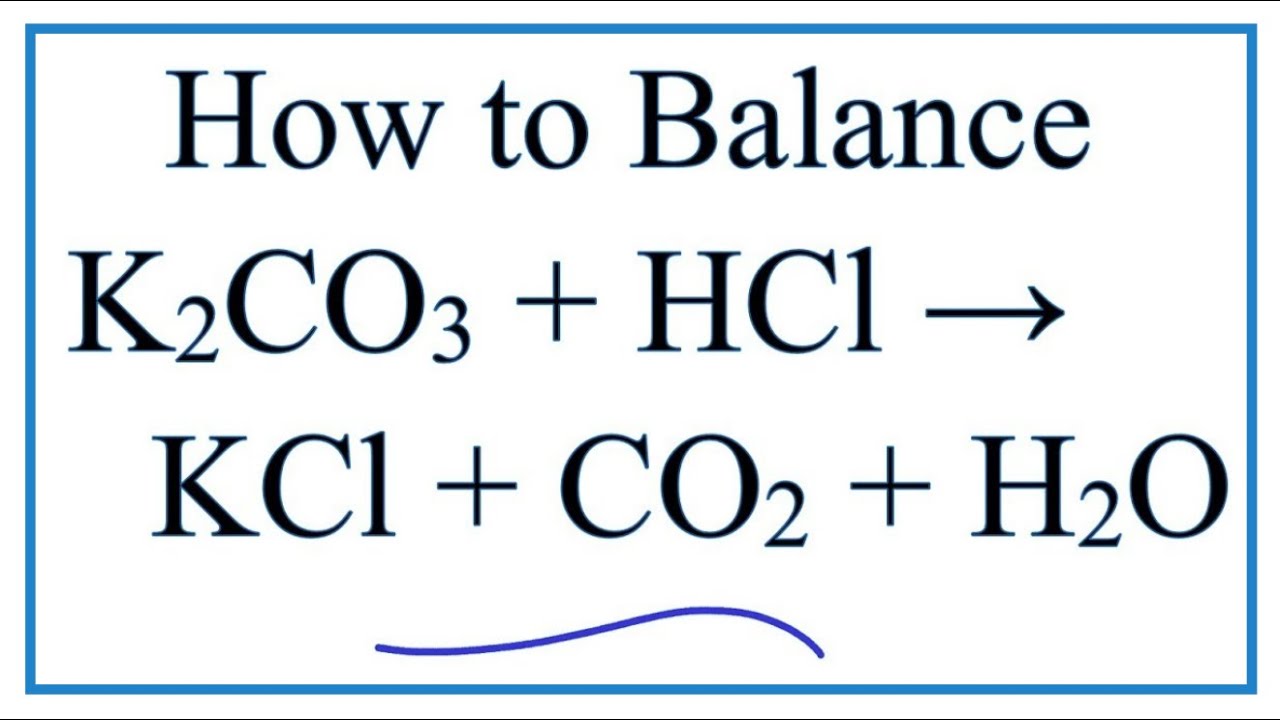
In the reaction between potassium carbonate and hydrochloric acid, 25 grams of salt were produced. If excess acid was available, how many grams of the carbonate were used? - Quora
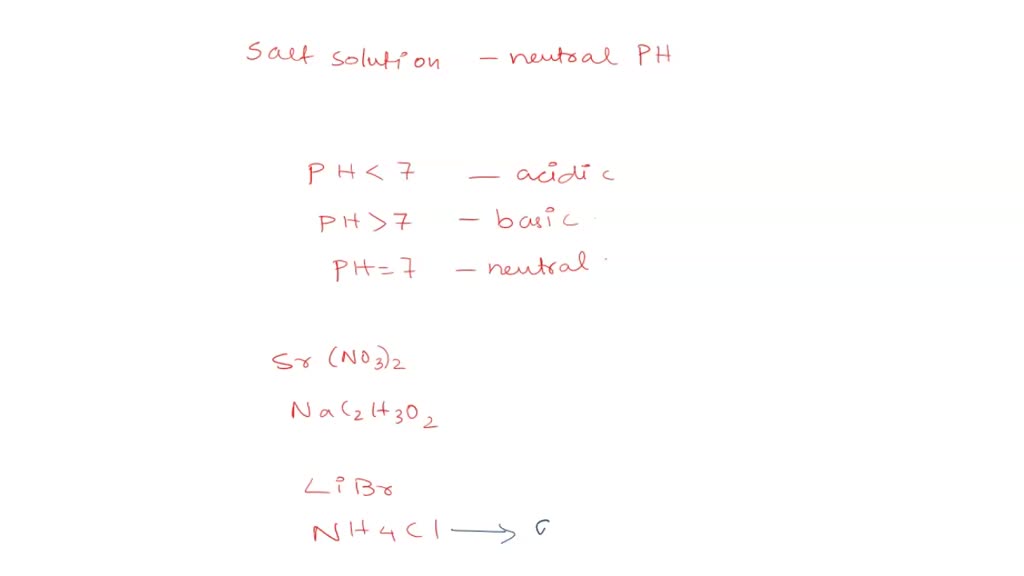
SOLVED: Select the salt from the list below which will produce a basic aqueous solution. Group of answer choices NaNO3 K2CO3 NH4Cl K2SO4
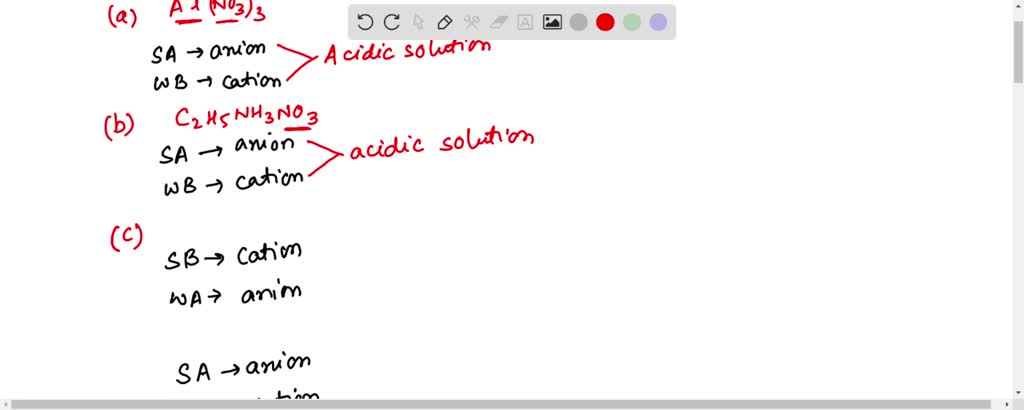
SOLVED: Determine if each salt will form a solution that is acidic, basic, or pH-neutral. a. Al(NO3)3 b. C2H5NH3NO3 c. K2CO3 d. RbI e. NH4ClO