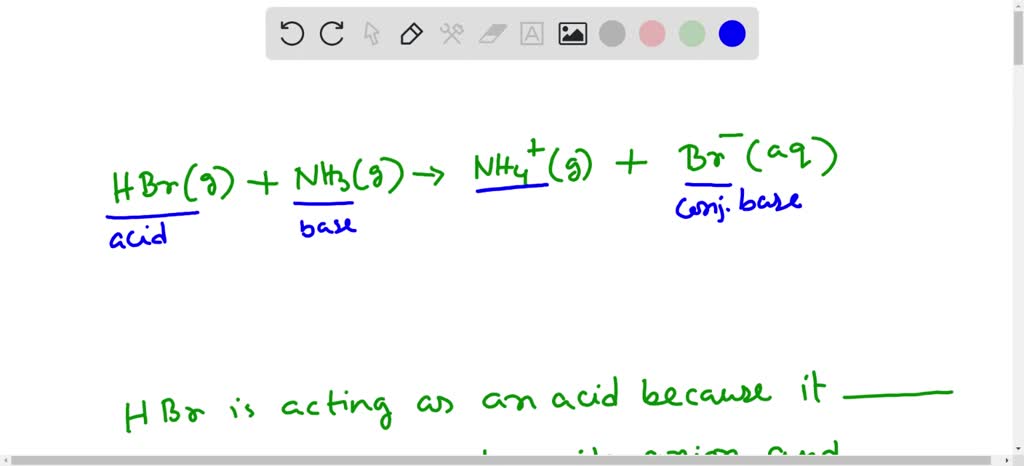
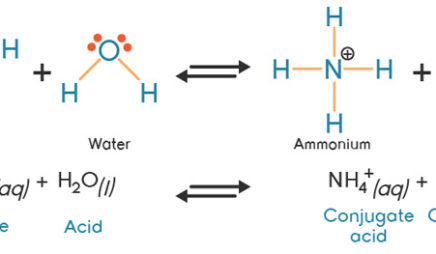
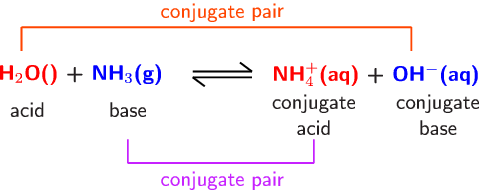
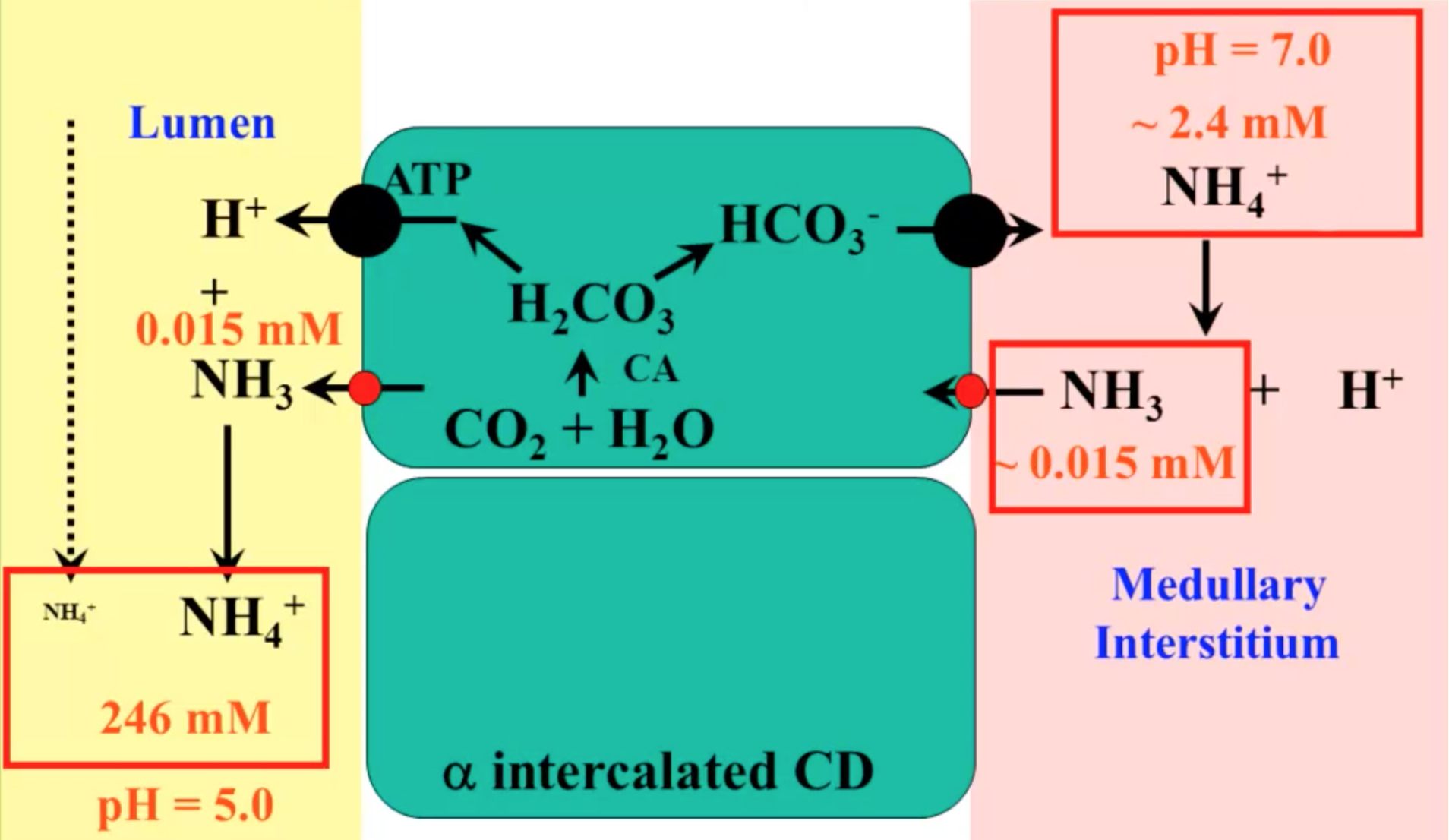
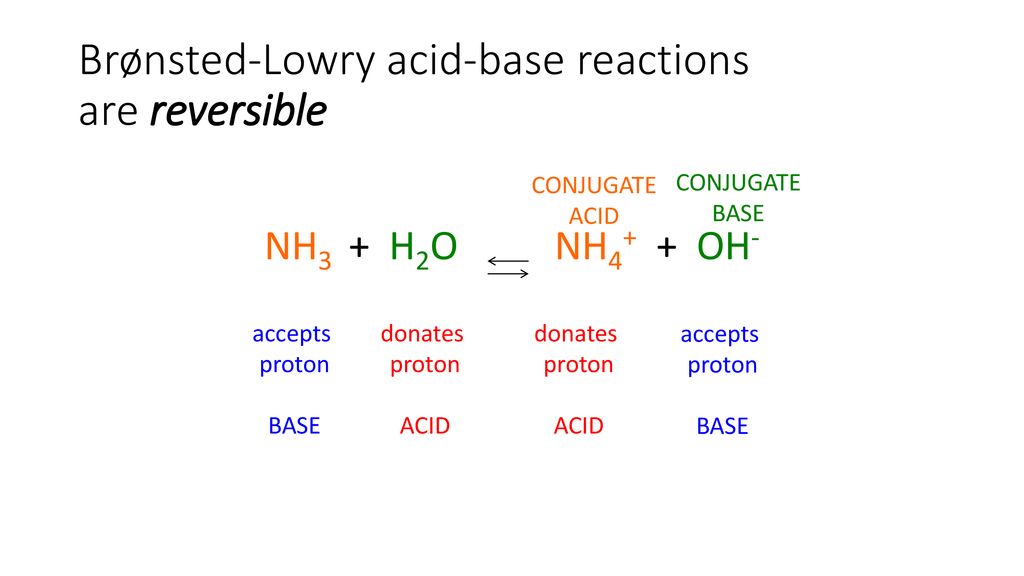
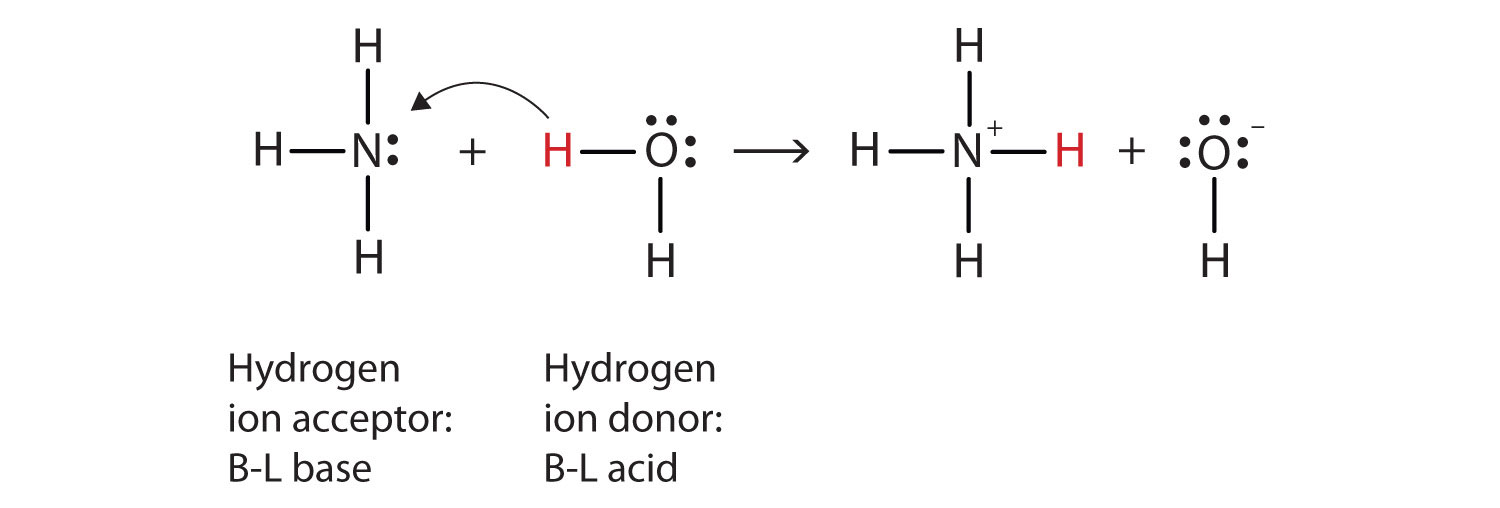
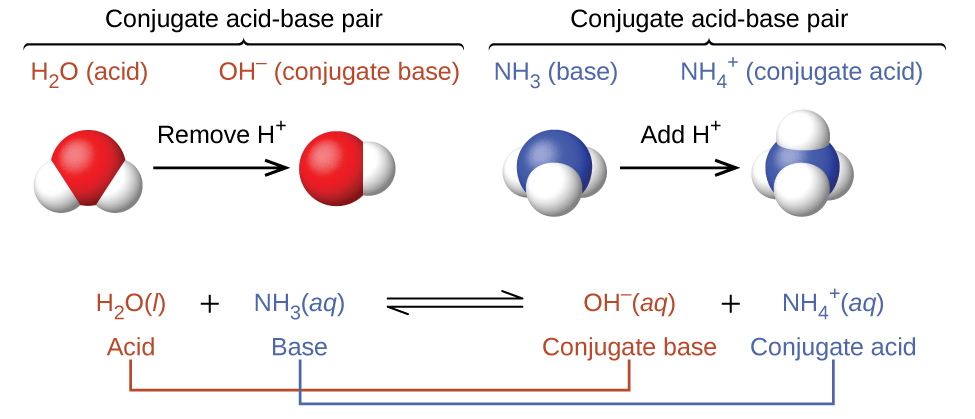
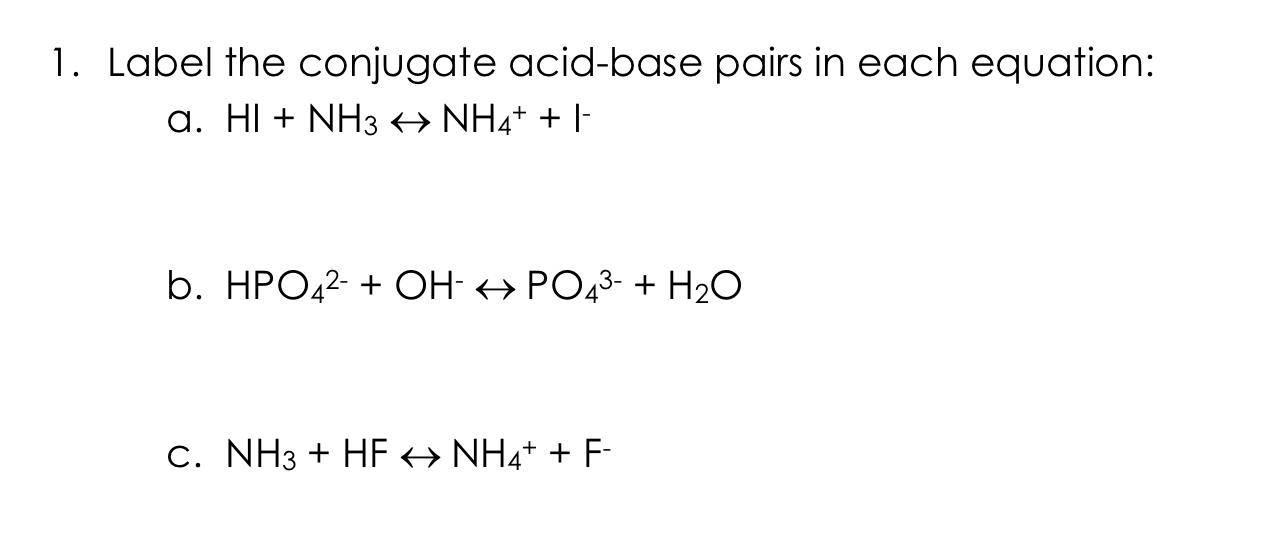Identify the conjugate acid-base pairs in this equilibrium. NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com
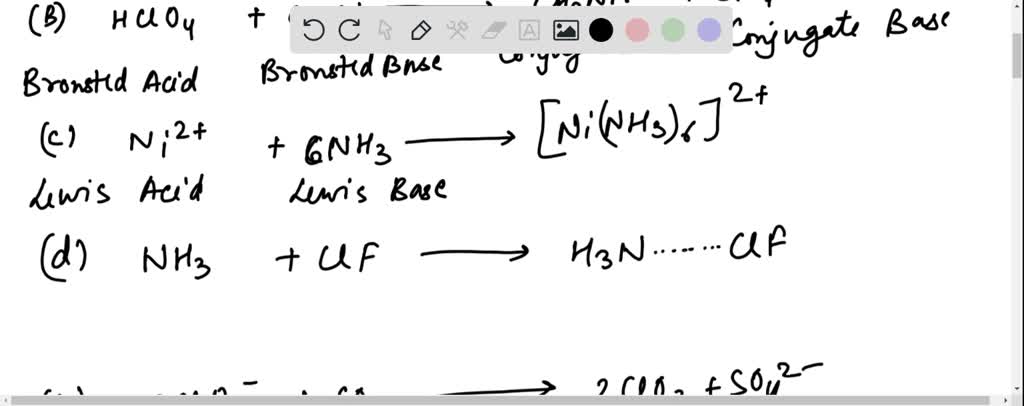
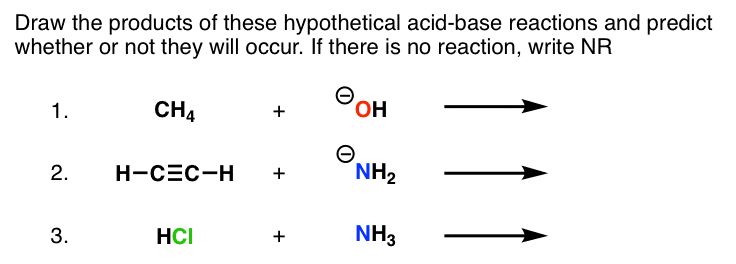
SOLVED: For each of the following reactions, identify the acid and the base by circling the acid and boxing the base. Also indicate whether Lux-Flood, Lewis or Bronsted-Lowry is the most appropriate

⚗️HELP In the following acid-base reaction, NH4+ is the H2PO4- (aq) + NH3(aq) → HPO42- (aq) + - Brainly.com

Spontaneity of the acid–base reaction between acetic acid and ammonia... | Download Scientific Diagram

organic chemistry - Why In This Reaction Acetic Acid is strong acid and NH3 is strong base ?please explain in details and thanks for answer - Chemistry Stack Exchange