Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa
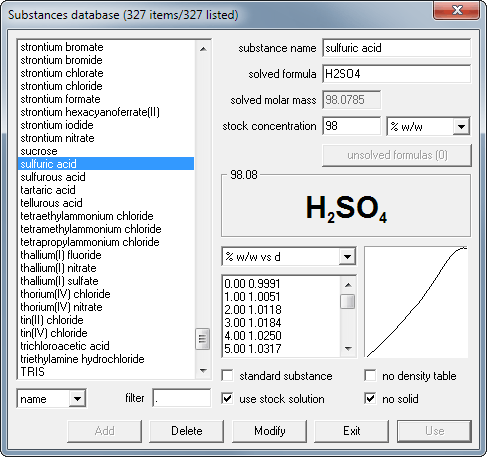
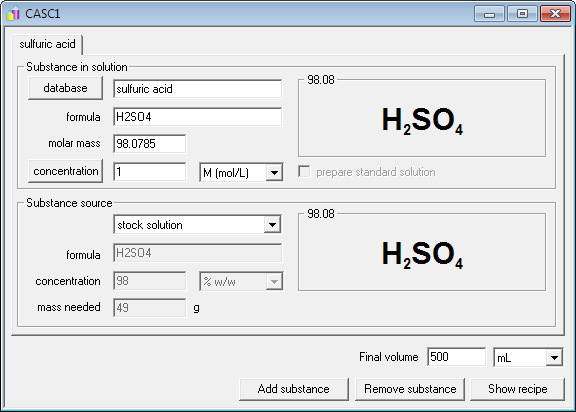
How to prepare 500 mL of 10-1 N H2SO4 solution (Mr = 98.00) from a 50.50% (g/ g) solution with a density of 1,400 g/ mL - Quora

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

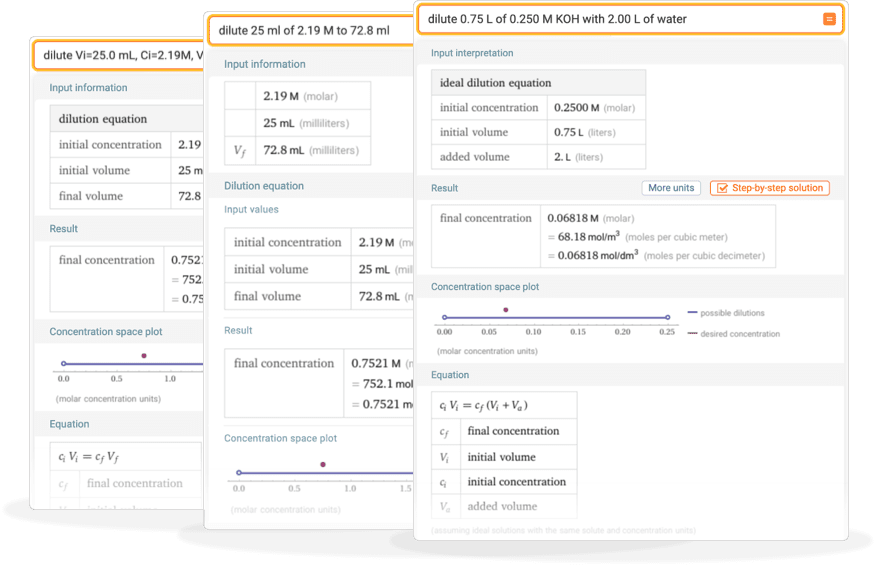
Calculating molarity units molar concentration of solutions practice questions on molarity how to make up a standard solution how to determine solubility gcse chemistry igcse KS4 science A level GCE AS A2
![SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and](https://cdn.numerade.com/ask_previews/5338532b-72ac-4610-a96e-d01f0328e5e1_large.jpg)
SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and
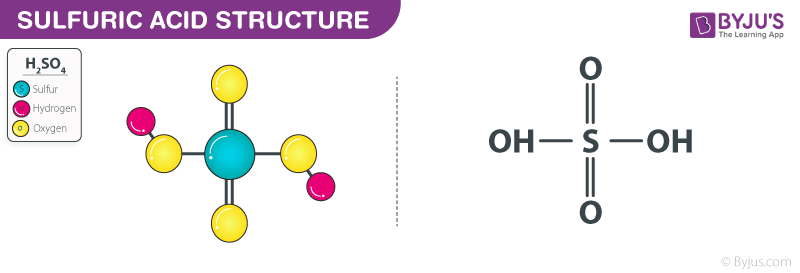
![SOLVED: Calculate [H3O+], [HSO4–] and [SO42–] in 0.20 M H2SO4. B. Calculate [H3O+], [HSO4–] and [SO42–] in 0.020 M H2SO4. Hint: Is the assumption that [HSO4–] = [H3O+] valid? SOLVED: Calculate [H3O+], [HSO4–] and [SO42–] in 0.20 M H2SO4. B. Calculate [H3O+], [HSO4–] and [SO42–] in 0.020 M H2SO4. Hint: Is the assumption that [HSO4–] = [H3O+] valid?](https://cdn.numerade.com/ask_previews/6ce3e047-4e7c-4aae-adf9-48149df80471_large.jpg)
SOLVED: Calculate [H3O+], [HSO4–] and [SO42–] in 0.20 M H2SO4. B. Calculate [H3O+], [HSO4–] and [SO42–] in 0.020 M H2SO4. Hint: Is the assumption that [HSO4–] = [H3O+] valid?

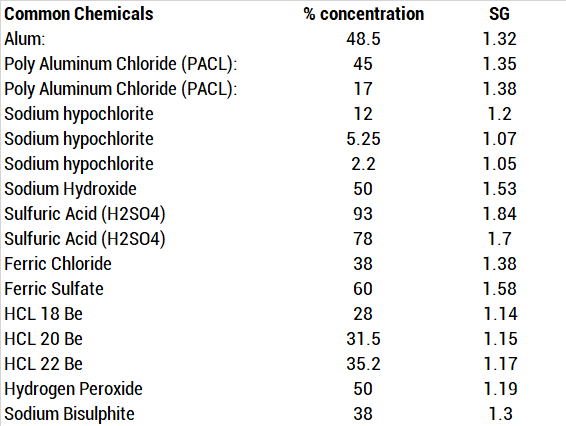




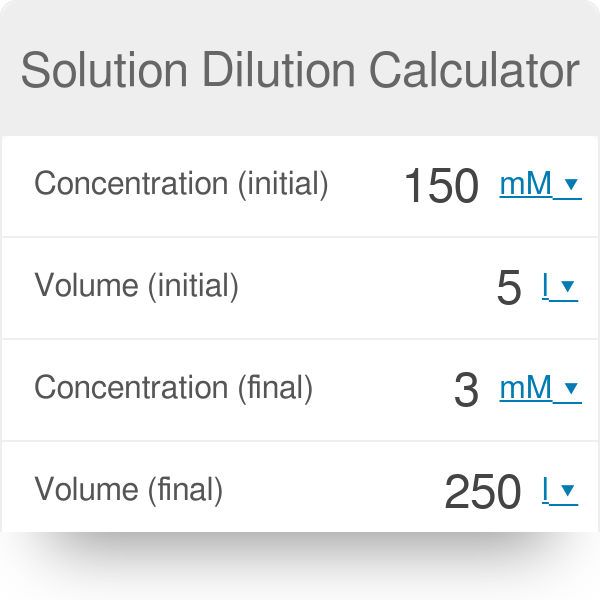


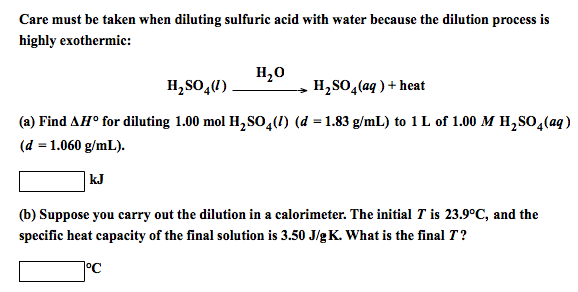


:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)