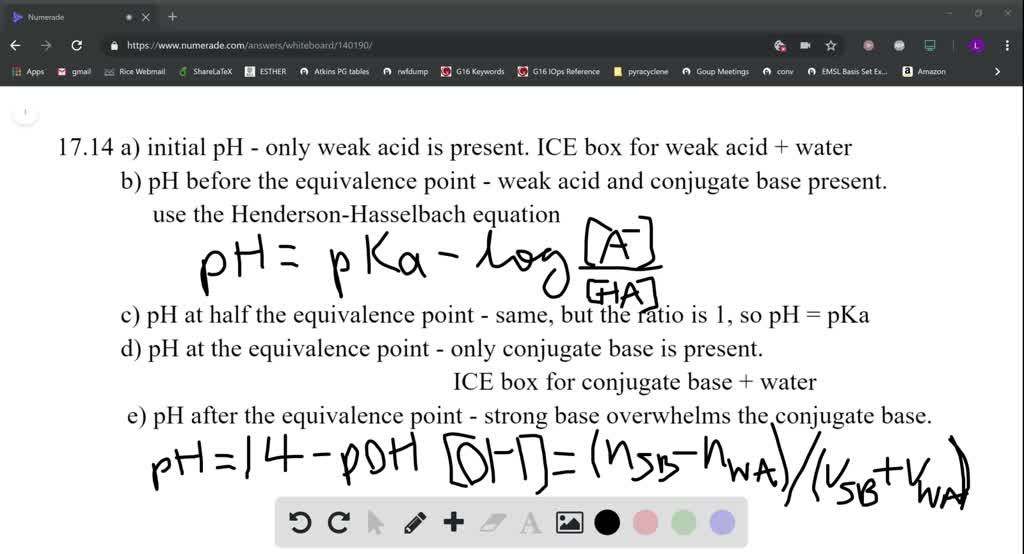
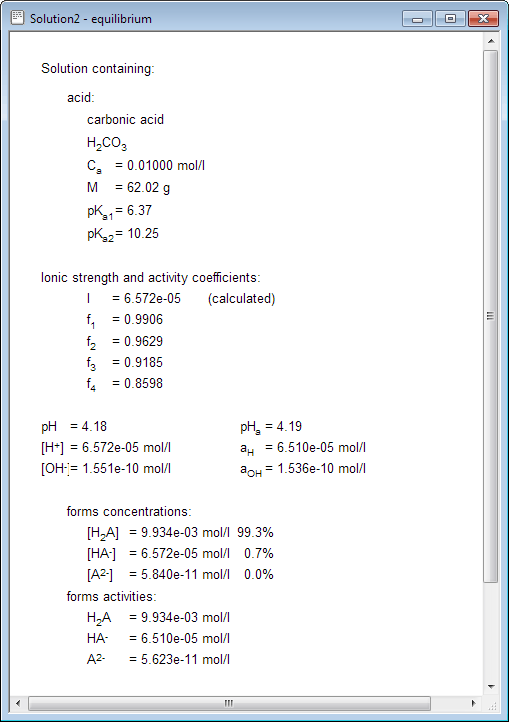
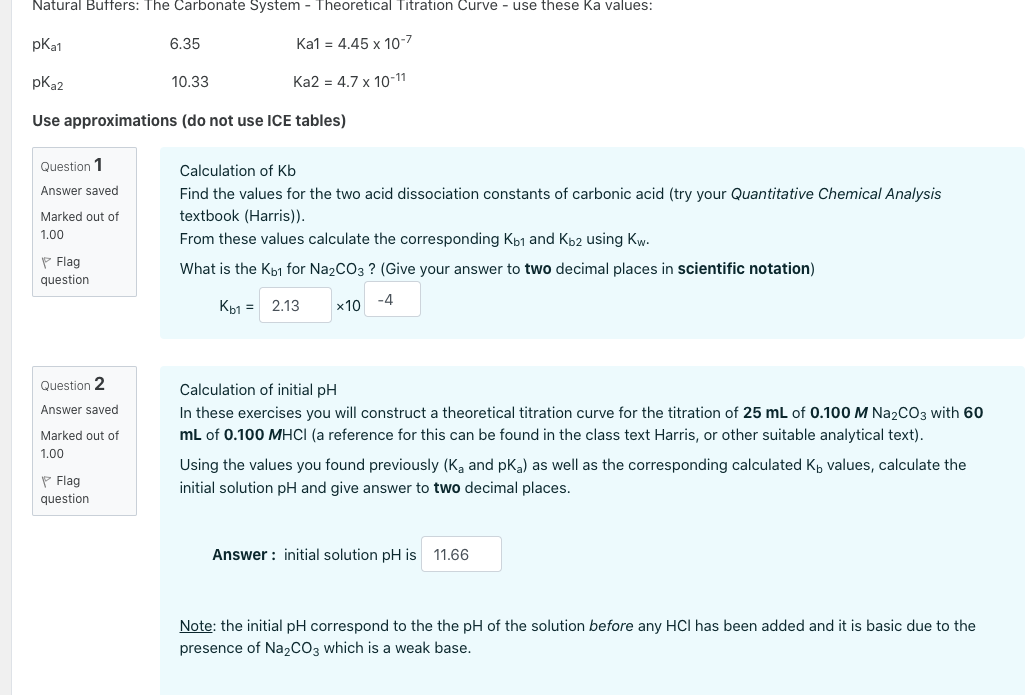
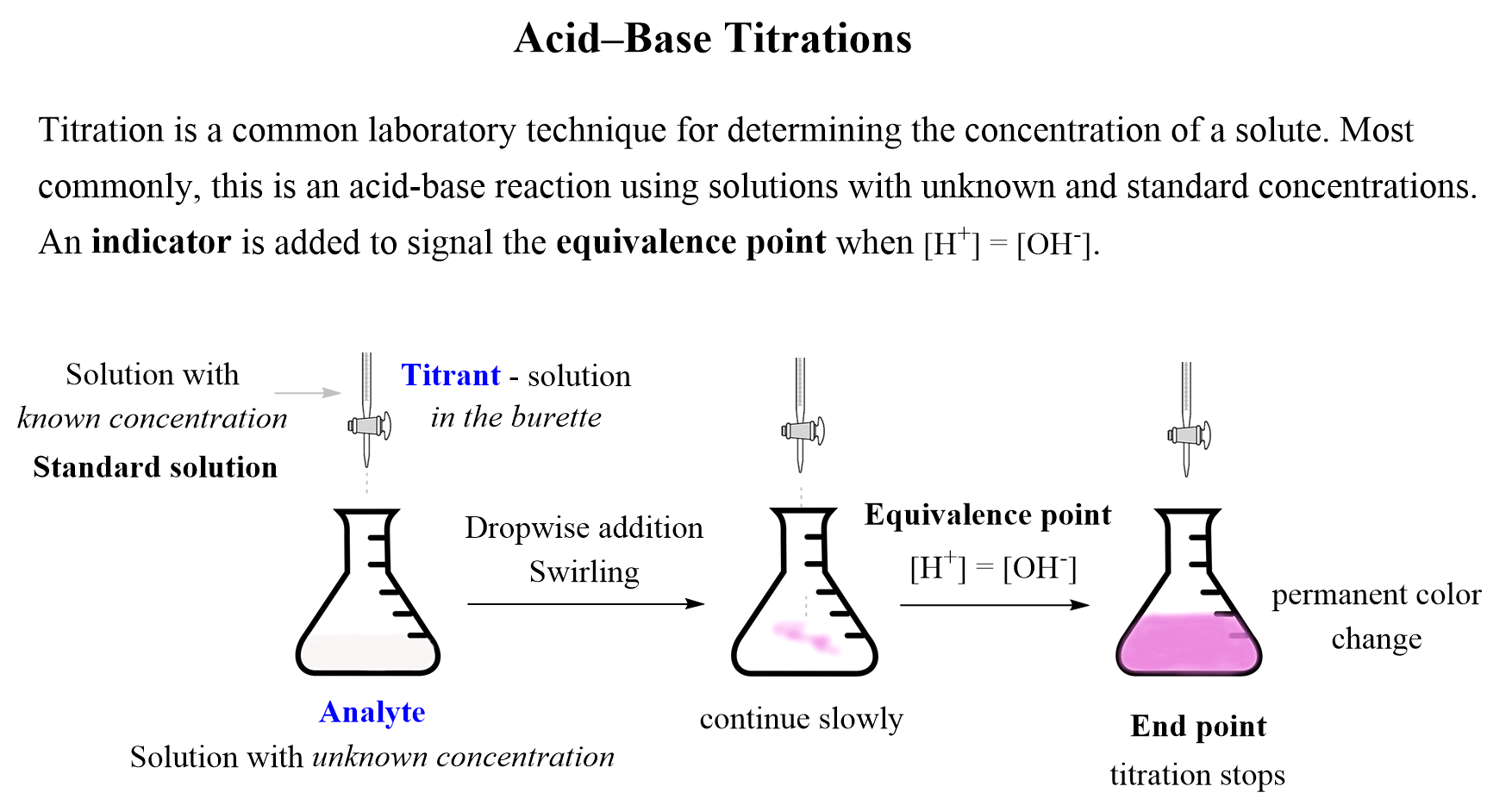
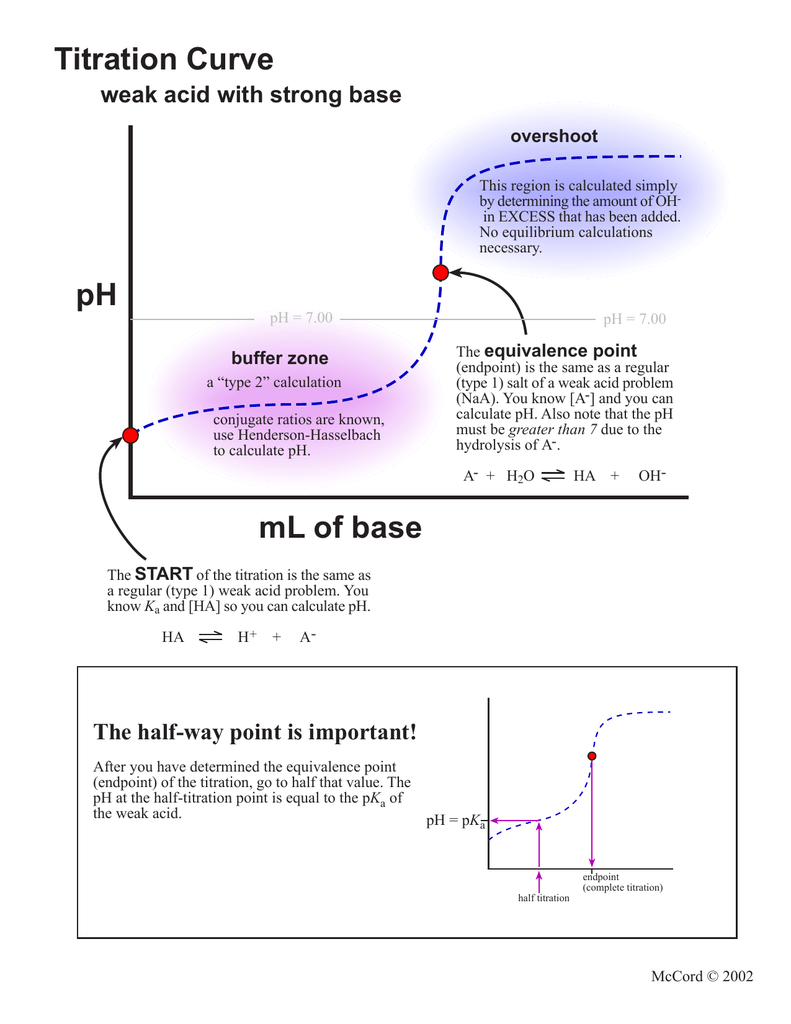
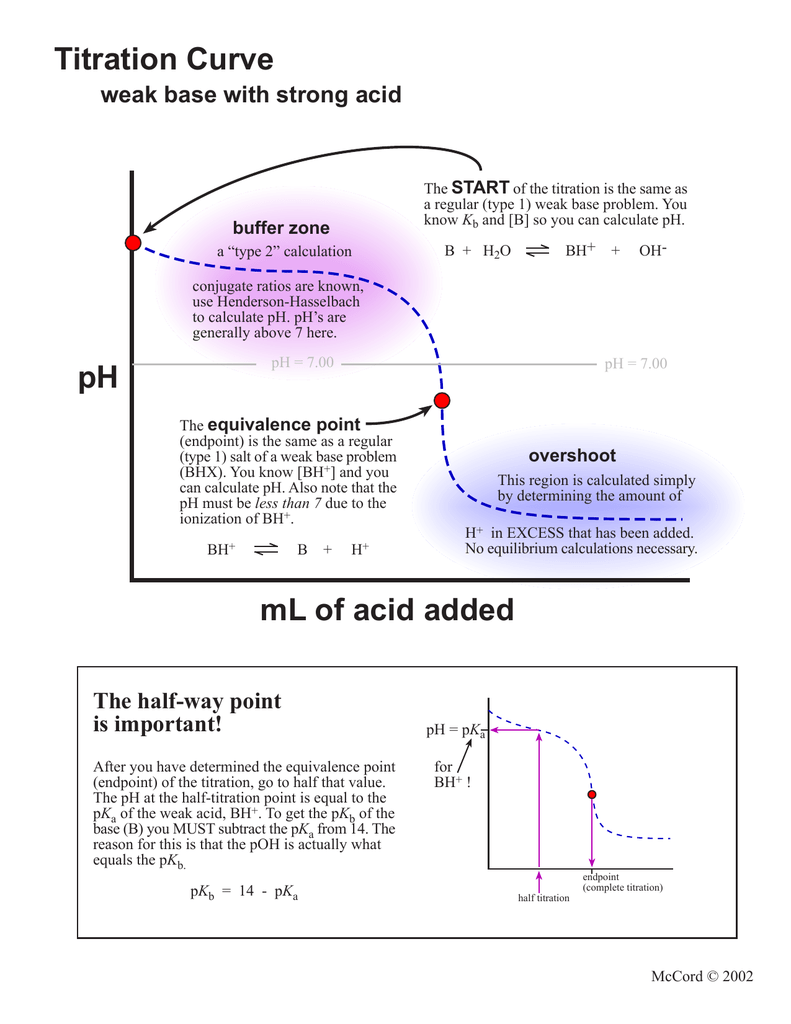
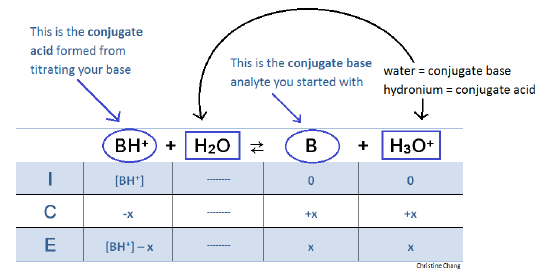
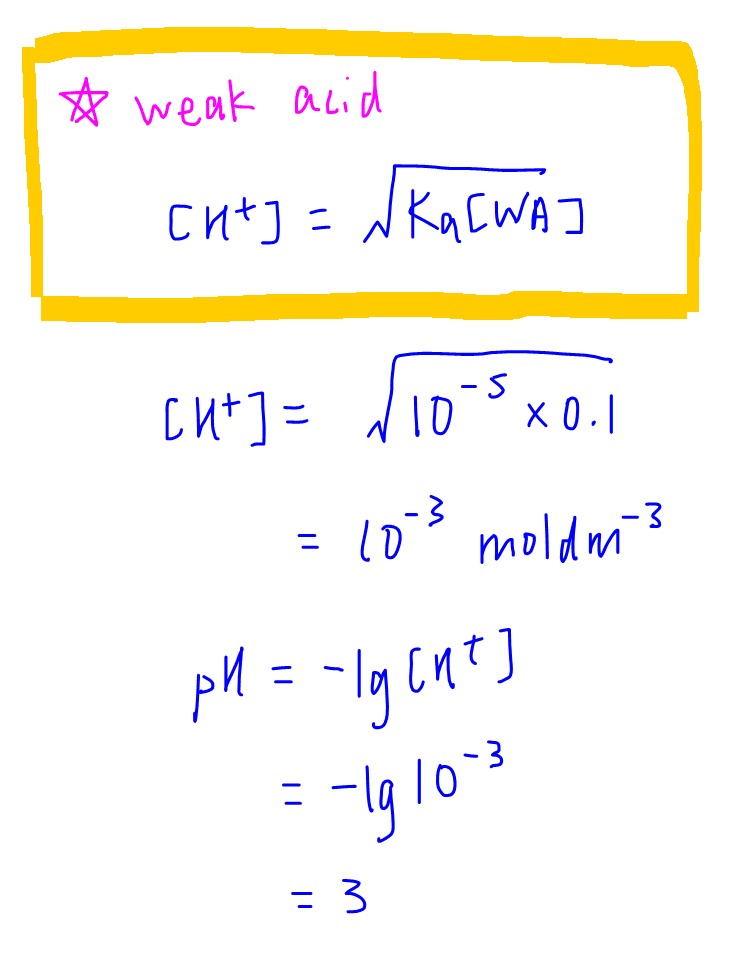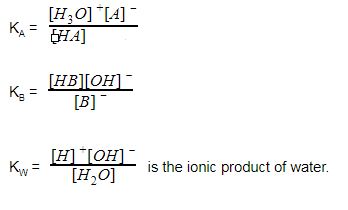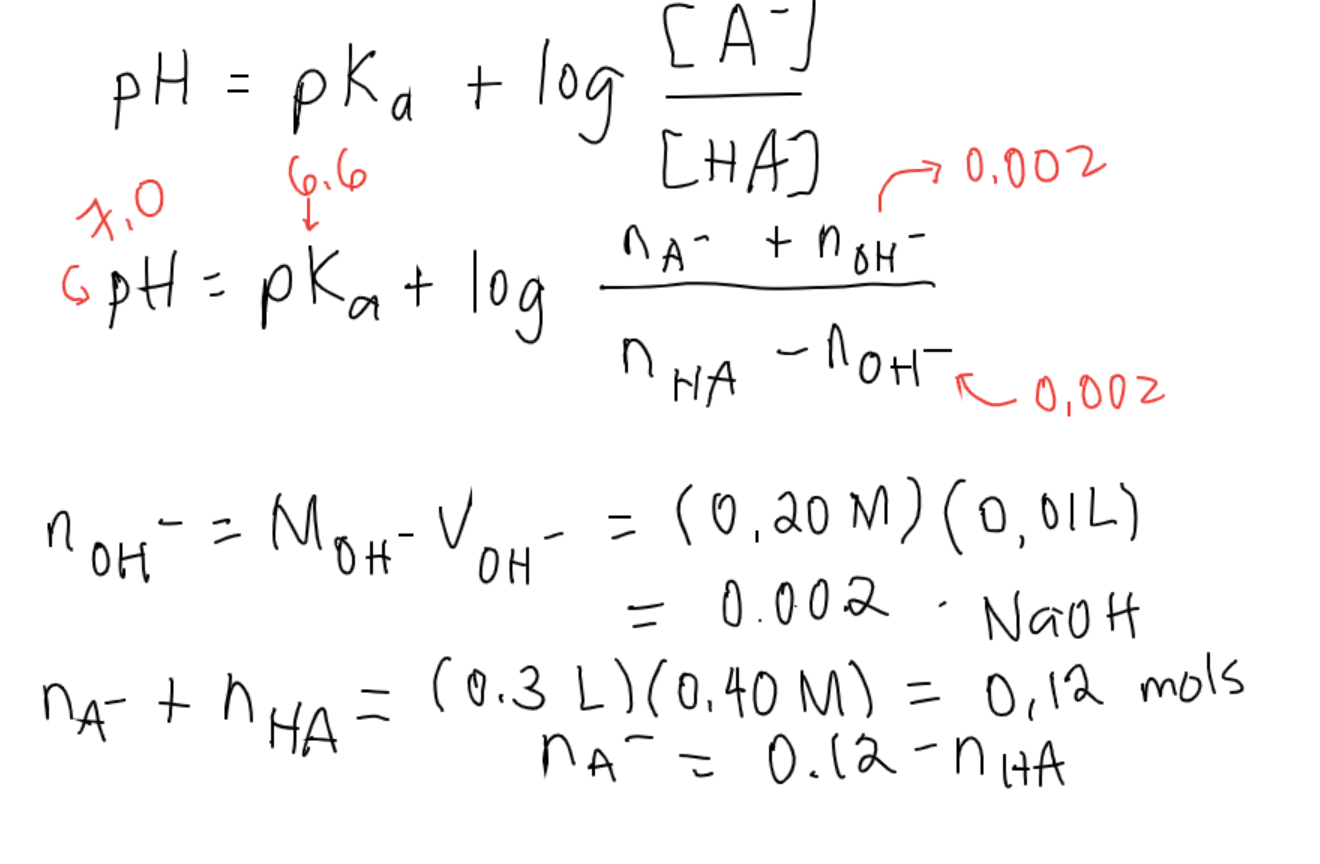25 ml of 0.1 M acetic acid is titrated with 0.1NaoH solution. The pH of the solution atequivalence point will be (log 5 0.7CH3COOH = 4.76)

University of South Carolina Board of Trustees Calculate the pH in the titration of mL of M HCl with M NaOH after a) 0.00 mL strong. - ppt download